Review Article
Thermo Reversible Buffered Tenofovir Nano Gel for Vaginal Delivery
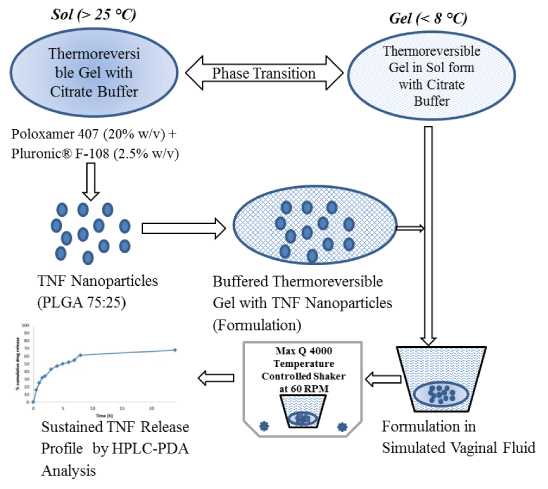
This study was designed to develop buffered thermoreversible gel containing tenofovir (TNF) loaded PLGA nanoparticles for sustained drug release and prolonged protection from HIV-1 sexual transmission. Optimized buffered thermoreversible formulation containing TNF loaded PLGA nanoparticles was in solution form at 2-8oC and converted into a gel matrix at 28oC. PLGA nanoparticles were prepared by two methods; (a) Solvent diffusion method, (b) Emulsification- solvent evaporation and effective particles diameter was 94.3-228 nm and 265-413 nm respectively by dynamic light scattering technique. Morphology of the particles was analyzed by scanning electron microscope and micro image showed smooth & spherical in shape nanoparticles. Triton® X-100 a non-ionic surfactant effectively reduced the particle size of nanoparticles in solvent diffusion method but the desired TNF encapsulation was not observed. Drug encapsulation and drug loading were analyzed in nanoparticles prepared by emulsification-solvent evaporation and the values were 1.43-10.78% and 0.25-1.18% respectively. In-vitro release of TNF from Buffered thermoreversible gel containing PLGA nanoparticles was conducted in simulated vaginal fluid (pH 4.2). In 8 h, cumulative % drug release was 61% and after 24 h it was 67.75%. Drug release mechanism was diffusion and erosion controlled. Results demonstrated the therapeutic potential of the vaginal nano gel formulation for prolonged protection against HIV-1 transmission as an STD.
Keywords
Tenofovir; Thermoreversible; Gel; PLGA nanoparticles; Triton X-100
Introduction
Tenofovir is an antiretroviral drug known as nucleotide analogue reverse transcriptase inhibitors, which block reverse transcriptase, an enzyme crucial to viral production in HIV-infected people [1]. It is currently considered the preferred first line treatment for HIV because of its potency, overall low toxicity, and convenience of dosing [2]. This drug is administered orally in the form of disoproxil ester, which is deesterified to achieve a bioavailability of more than 20% [3] This prodrug has a plasma stability issue and non-specific distribution. It is also reported that parenteral administration showed nephrotoxicity [4,5].
Literature revealed that PLGA, is a FDA-approved and widely accepted biodegradable copolymer used in nanoparticle formulation, which can also provide the sustained release of an encapsulated drug. Application of FDA approved excipients may feasible to clinical testing of final formulation. Designs of nanoparticles encapsulated drugs are still challenging area for the formulation scientists. Nanoparticles could be site specific delivery by size dependent uptake by affected cell and it may also improve drug efficacy by release the drug from the nanoparticles over a prolonged time [6]. Zhang et al. [7] studied the pH responsive PLGA/ methacrylic acid copolymer (Eudragit® S-100, or S-100) nanoparticles having TNF and results suggested the alternative controlled drug delivery system for intravaginal delivery TNF. Another formulation based on lipid were studied by Xu et al. [8] and reported that Liposomes have low encapsulation efficiency problem.
Nano size based formulation in suspension form may face the problem of low retention time at desired site that ultimately will lead to poor bioavailability. Therefore we hypothesized that if we can increase the retention of nanoparticles by gel matrix at vaginal site that may improve efficacy of TNF. This study was designed to develop thermoreversible gel containing TNF loaded PLGA nanoparticles. Final formulation will be in solution form at 2-8oC and covert into gel matrix dispersed TNF loaded PLGA nanoparticles.
Materials and methods
Tenofovir was purchased from Waterstonetech, LLC, USA. 75:25 poly(DL-lactide-co-glycolide) (PLGA) was purchased from DURECT, USA. Ethyl acetate, Pluronic®F-68(PEG-PPG-PEG), Poloxamer 407, Pluronic® F-108 and poly (vinyl alcohol) 98-99% hydrolyzed were purchased from Sigma-Aldrich, USA. Sodium phosphate monobasic anhydrous USP, HPLC water, HPLC grade acetonitrile were purchased from Fisher scientific, USA. Sodium phosphate dibasic heptahydrate was purchased from Affymetrix, Inc, USA. Millex® -GV, syringe driven filter unit Durapore® (PVDF) 0.22 µm membrane was purchased from Millipore, USA
Preparation of TNF loaded PLGA nanoparticles by solvent diffusion
Briefly, TNF (10-100 mg) and the polymer (100–400 mg), PLGA were co-dissolved in 5 ml DMSO. The organic phase was added drop wise (2ml/min.) to aqueous phase, containing Triton X 100, under homogenization at 4000-5000 rpm for 30 min. (Power Gen 1000, fisher scientific, USA). Suspension was ultrasonicated for 5-10 minutes and ultra-centrifuged at 15000 rpm for 90 min (Beckman L8-70 M Ultra-centrifuge, Brea, CA, USA) to collect NPs and then washed with distilled water to remove the surfactant. Settled pallet was re-suspended in distilled water and tested for particles size by DLS and SEM.
Preparation of TNF loaded PLGA nanoparticles by emulsification-solvent evaporation
TNF-loaded PLGA nanoparticles were prepared using the modified emulsion/solvent evaporation technique. PLGA (75:25) was dissolved in ethyl acetate and added to aqueous phase containing pluronic®F68 and TNF to prepare a primary emulsion and then it was ultrasonicated (Pulse: 0.9/0.1;amplitude 30-35%) for 10 minutes on ice batch. Primary emulsion added to secondary aqueous phase (2% w/v PVA solution) and ultrasonicated for 5 minutes than ethyl acetate evaporated at room temperature by constant magnetic stirring for 12-18 h. The prepared nanoparticles were separated by ultracentrifuge and supernatant discarded and settled pellets were collected in lyophilization vial and lyophilized at -50oC for 24-48 h.
Preparation of thermo reversible buffered gel containing PLGA nanoparticles
Poly(ethylene glycol)-block-poly(propylene glycol-block-poly(ethylene glycol) (20% w/v) was added in 4.2 citrate buffer and stored at 2-8oC temperature for 24 hour for complete dissolution than 2.5% w/v of Pluronic® F-108 (poly(ethylene glycol-block-poly(propylene glycol-block poly(ethylene glycol) added to it and stored further 12 h. Liquid slight viscous solution was obtained. This solution was checked for gel formation by gradually increasing temperature in water bath and observed visually. Dried TNF loaded PLGA nanoparticles were added to this solution and stored in freeze until further use.
Nanoparticle characterization
Particle size determination: The particles size and distribution of TNF loaded PLGA nanoparticles were measured by dynamic light scattering technique using Brookhaven 90plus, USA at 25oC. Particles size was analyzed before lyophilization of the batches and represented as effective diameter in nm.
Morphology: The micro scaled image was taken by scanning electron microscope. The lyophilized powder was sprinkle on carbon tap stick stub than it was coated by gold and focused under microscope under vacuum and 5KV than focused images was captured at different magnification.
Encapsulation efficiency (EE) and drug loading (DL): EE & DL were determined by RP-HPLC method. 10 mg of lyophilized TNF loaded PLGA nanoparticles were dissolved in 2 ml ethyl acetate after dissolving completely, 1 ml HPLC water added and mix well and ultrasonicated for 1 min. for drug extraction and dissolution. Solvent was evaporated stirring overnight at room temperature. The sample filtered by 0.22 µm Durapore (PVDF) membrane filter than injected by auto sampler and TNF concentration was calculated by AUC of peak using standard curve. The %EE & %DL were calculated by using following formulas:
%Drug loading :( Drug in nanoparticles /Weight of nanoparticles) X 100
% Encapsulation efficiency: (Total drug in nanoparticles /Total drug taken) X 100
In vitro drug release from PLGA nanoparticles dispersed in buffered gel: In vitro release of TNF from the PLGA (75:25) nanoparticles was conducted over 24 h using Simulated Vaginal fluid type 1 (SVF). 50 mg of PLGA nanoparticles (159.5 µg TNF) was added to 5 ml thermoreversible buffered gel and dispersed by shaking or using slight vortex than nanoparticles dispersed thermoreversible citrate buffered gel was added to 20 ml SVF as a release medium in a 50 ml tube than placed in shaker (MaxQ4000) at 37 °C with an agitation speed of 60 rpm. At predetermined time intervals, 1 ml of release medium and replaced with fresh medium. The amount of drug released was measured by RP-HPLC method at 260 nm. The samples was filtered by syringe driven Durapore (PVDF) 0.22 um membrane filter before injecting in to HPLC. The standard curve was prepared using mobile phase consisted of acetonitrile/50mM sodium phosphate buffer pH 5.12 at a ratio of (2.5:97.5; v/v). The flow rate was 1 ml/min. and injection volume was 20 µl. Linearity was obtained by 2-10 µg/ml (r2 0.9988) with calibration curve of Area = 49185Conc. + 865.86.
Results and discussion
Two different methods were applied for preparation of PLGA nanoparticles with TNF. In first method (Solvent diffusion) effective particle diameters of nanoparticles without and with TNF were 94.3-137 nm (PGSD1) and 207.8-228.2 nm (PGSD4) respectively with narrow size distribution (Table 1, Figure 1,2). The drawback of this method was found poor encapsulation of TNF with PLGA nanoparticles, when it was analyzed by RP-HPLC, no drug peak was detected. On the theoretical basis small amount of TNF should be encapsulated during nanoprecipitation, but during our studies we did not observed TNF at quantification level. Possible reason can be partitioning effect towards the aqueous phase during mixing. Another reason may be Triton X 100 that preventing TNF encapsulation because of strong surfactant effect. Therefore, we tried alternative method (emulsion-solvent evaporation) for water soluble drug molecules based on literature [9]. But the particle size was increased from 265 nm to 413 nm as compared to solvent diffusion method and PDI increased from 0.131 to 0.217. Lower particles size was obtained with increased ultra-sonication time [10]. Significant variation in particle size was not observed when Pluronic F68 concentration increased from 0.2 to 1% w/v in aqueous phase. Triton X 100, a non-ionic surfactant was effectively reduced particle size and PDI but TNF encapsulation was not detected in PLGA nanoparticles. Triton X 100 as a surfactant in PLGA nanoparticle preparation was first time tried by our lab and result showed particle size than 100 nm of PLGA nanoparticles that suggesting further use this surfactant with other polymers systems. We did not found any report for use of Triton X 100 in preparation of PLGA nanoparticles.
Table 1 Batch variables and Physiochemical parameters of PLGA nanoparticles.
Batch no. |
PLGA conc. (mg) |
TNF (mg) |
Triton X100 |
Pluronic F68 (%w/v) |
Sonication time (min.) |
Particle size (nm) |
PDI |
EE (%) |
DL (%) |
Zeta Potential (mV) |
PGSD1 |
100 |
0 |
0.5 |
0 |
0 |
97 |
0.074 |
- |
- |
- |
PGSD2 |
400 |
0 |
0.5 |
0 |
0 |
137.1 |
0.062 |
- |
- |
- |
PGSD3 |
400 |
10 |
0.5 |
0 |
0 |
207.8 |
0.110 |
- |
- |
- |
PGSD4 |
100 |
10 |
0 |
0.2 |
0 |
228.2 |
0.143 |
- |
- |
- |
PGDE1 |
100 |
10 |
0 |
0.2 |
5 |
413.6 |
0.237 |
10.78 |
1.18 |
- |
PGDE2 |
400 |
0 |
0 |
0.6 |
10 |
278 |
0.131 |
- |
- |
-5.86 |
PGDE3 |
400 |
100 |
0 |
0.6 |
10 |
298 |
0.217 |
1.18 |
0.31 |
-6.53 |
PGDE4 |
400 |
100 |
0 |
1 |
10 |
300 |
0.209 |
1.43 |
0.42 |
-5.76 |
Table 1 Batch variables and Physiochemical parameters of PLGA nanoparticles.
×
Figure 1 Diagrammatic representation of formulation design and release study.
Figure 2 Dynamic light scattering data of PLGA nanoparticles: Prepared by Solvent diffusion method (PGD1, PGSD3), and emulsification-solvent evaporation method (PGDE1, PGDE4).
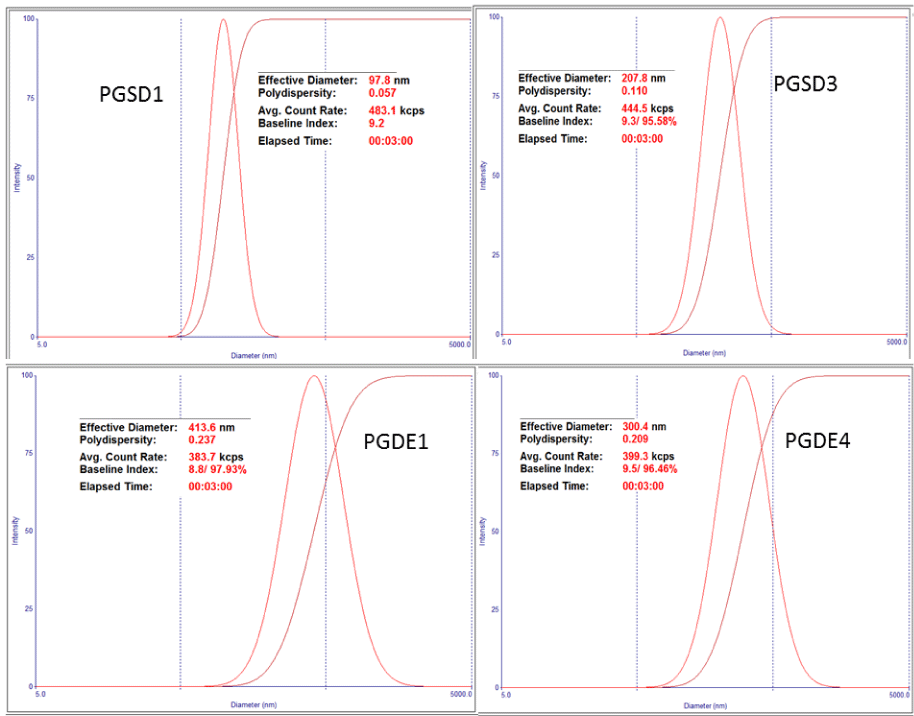

Figure 2 Dynamic light scattering data of PLGA nanoparticles: Prepared by Solvent diffusion method (PGD1, PGSD3), and emulsification-solvent evaporation method (PGDE1, PGDE4).
×
Encapsulation & drug loading was obtained in range 1.43-10.78 and 0.25-1.18% respectively. EE was not decreased significantly, when drug concentration increased from 10 mg to 100 mg with 400 mg of PLGA [7]. Double emulsification method is commonly applied technique for encapsulation water soluble drugs in nanoparticles but our results showed low encapsulation of TNF because of the drug rapid partitioning to the external aqueous phase [11, 12].
The thermoreversible gel containing TNF loaded PLGA nanoparticles were developed for vaginal delivery. Poloxamer 407 (20% w/v) & Pluronic® F-108 (2.5% w/v) in combination showed gel matrix formation in between 28-30oC temperature range and reverse into solution form when placed at 2-8oC in refrigerator. Addition of Pluronic®F-108 was increased gelling temperature and viscosity of solution [13,14]. So it can be useful to optimize the gelling temperature close to the body temperature.
SEM image of PLGA nanoparticles showed smooth and spherical in shape (Figure 2). The particles were attached with other because of aggregation during lyophilization. Particles were seen properly when they coated with gold before focusing under electron microscope. The dark spot was observed when high voltage electron beam was applied on PLGA nanoparticles. It may be due to degradation of PLGA when exposed to high energy beam.
In-vitro release of TNF in SVF conducted for 24 h (Figure 3,4). First half an hour release was 15% that may be because of local bust release from the outer gel matrix. In 8 h, cumulative % drug release was 61% and after 24 h it was 67.75%. In-vitro release data is clearly indicating sustained release profile over the 24 h. Gel matrix mass was reduced over the 24 h release study that showed matrix erosion. Drug Release kinetics such as zero order, first order, and Higuchi model were fitted to release profile and R2 value was obtained 0.852, 0.961, and 0.982 respectively. Higuchi model shows that drug released by diffusion mechanism. Korsmeyer-Peppas model fitted to release profile, n-value was 0.45. Peppas model showed Non -Fickian transport that is indicative of the release mechanism from diffusion and erosion controlled process [9].
Figure 3 SEM micro images of PLGA nanoparticles (A, B without gold coating) by Solvent diffusion method, and (C, D with gold coating) by emulsification-solvent evaporation method (C, D).
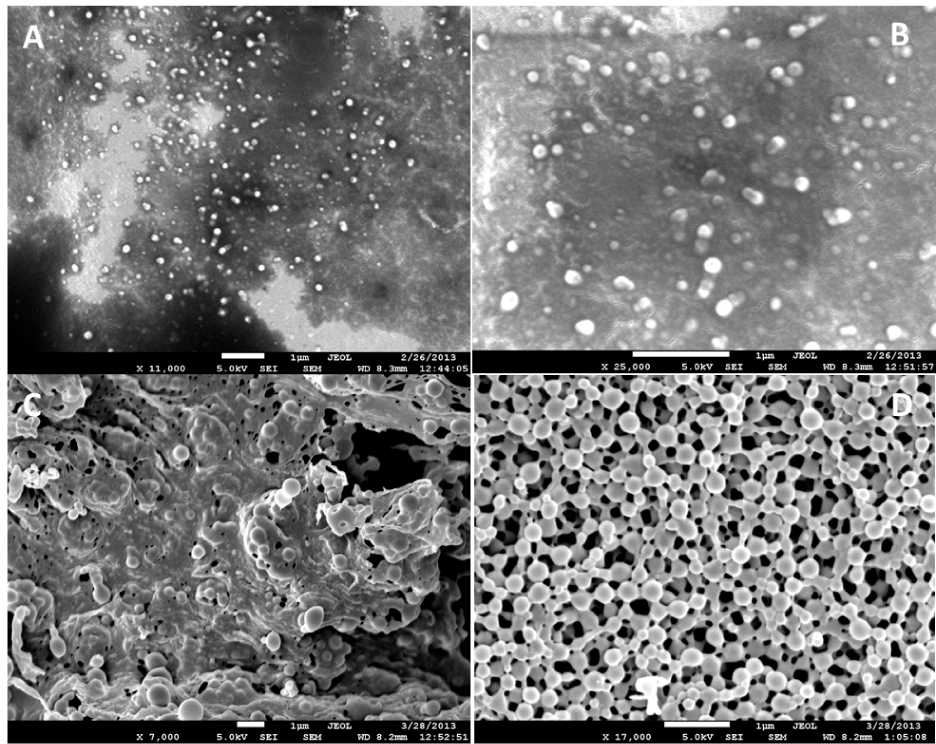

Figure 3 SEM micro images of PLGA nanoparticles (A, B without gold coating) by Solvent diffusion method, and (C, D with gold coating) by emulsification-solvent evaporation method (C, D).
×
Figure 4 In vitro release profile of thermoreversible gel containing tenofovir loaded PLGA nanoparticles.
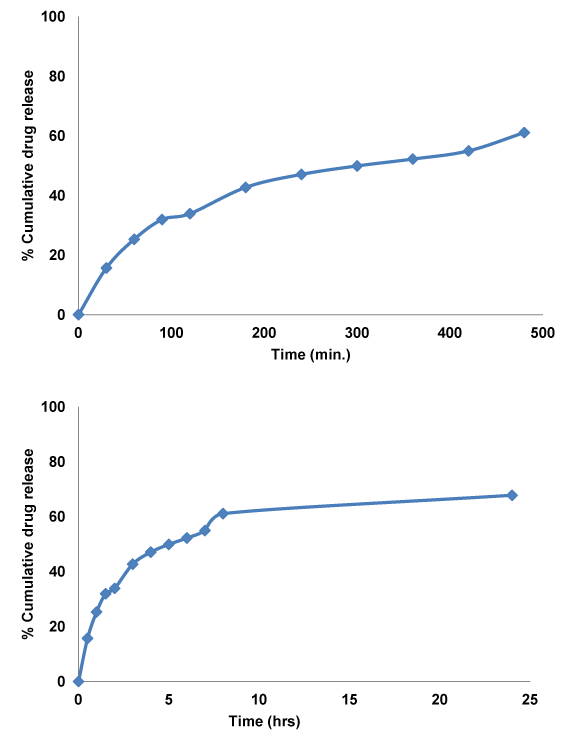

Figure 4 In vitro release profile of thermoreversible gel containing tenofovir loaded PLGA nanoparticles.
×
Conclusion
Both preparation methods were efficient to prepare PLGA nanoparticles. Results revealed that emulsification-solvent evaporation method has advantage over the solvent diffusion method for encapsulation of TNF but particles size was increased as compare to solvent diffusion method. Thermoreversible solution dispersed TNF loaded PLGA nanoparticles was slight viscous at 2-8oC temperature and have capability to converted to gel matrix at 28-30o temperature. More concentration variation in combination of Poloxamer 407 & Pluronic® F-108 can help to optimize gelling temperature close to vagina temperature. Sustained drug release behavior was showed by in-vitro release data and drug release mechanism was diffusion and erosion controlled. This study suggested that possibility of this formulation for vaginal drug delivery of anti HIV drugs. Further studies are required to optimize formulation with enhanced encapsulation, stability and in vivo efficacy in animal models. Additionally, it is required to generate toxicity & safety data.
Acknowledgements
This project has been funded in whole or in part with Federal funds (UL1TR000101 previously UL1RR031975) from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through the Clinical and Translational Science Awards Program (CTSA), a trademark of DHHS, part of the Roadmap Initiative, "Re-Engineering the Clinical Research Enterprise.
References
- Hawkins T, Veikley W, St Claire RL 3rd, Guyer B, Clark N, Kearney BP. Intracellular pharmacokinetics of tenofovir diphosphate, carbovir triphosphate, and lamivudine triphosphate in patients receiving triple-nucleoside regimens. J Acquir Immune Defic Syndr. 2005; 39: 406-11.
- Tenofovir. Leading HIV Medication, Linked with Risk of Kidney Damage. 2012; 415-221.
- Azanza JR, García Quetglas E, Sádaba B, Gómez-Giu A. Tenofovir: pharmacology and interactions. Enferm Infecc Microbiol Clin. 2008; 26: 2-6.
- Gitman MD, Hirschwerk D, Baskin CH, Singhal PC. Tenofovir-induced kidney injury. Tenofovir-induced kidney injury. 2007; 6: 155-64.
- James C, Steinhaus M, Szabo S, Dressler R. Tenofovir-related nephrotoxicity: case report and review of the literature. Pharmacotherapy. 2004; 24: 515-518.
- Farokhzad OC, Cheng J, Teply BA, Sherifi I, Jon S, Kantoff PW, et al. Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc Natl Acad Sci U S A. 2006; 103: 6315-20.
- Zhang T, Sturgis TF, Youan BB. pH-responsive nanoparticles releasing tenofovir intended for the prevention of HIV transmission. Eur J Pharm Biopharm. 2011; 79: 526–536.
- Xu X, Khan MA, Burgess DJ. A quality by design (QbD) case study on liposomes containting hydrophilic API: I formulation, processing design and risk assessment. Int J Pharm. 2011; 419: 52-59.
- Amjadi I, Rabiee M, Hosseini MS, Mozafari M. Synthesis and characterization of Doxorubicin-loaded Poly(Lactide-co-glycolide) nanoaprticles as a sustained release anticancer drug delivery system. Appl Biotechnol. 2012; 168: 1434-1447.
- Cohen-Sela E, Chorny M, Koroukhov N, Danenberg HD, Golomb G. A new double emulsion solvent diffusion technique for encapsulating hydrophilic molecules in PLGA nanoparticles. J Control Release. 2009; 133: 90–95.
- Tewes F, Munnier E, Antoon B, Ngaboni Okassa L, Cohen-Jonathan S, Marchais H, et al. Comparative study of doxorubicin-loaded poly(lactide-co-glycolide) nanoparticles prepared by single and double emulsion methods. Eur J Pharm Biopharm. 2007; 66: 488-92.
- Azizi M, Farahmandghavi F, Joghataei M, Zandi M, Imani M, Bakhtiary M, et al. Fabrication of protein-loaded PLGA nanoparticles: effect of selected formulation variables on particle size and release profile. J Polym Res. 2013; 20:110-124.
- Aka-Any-Grah A, Bouchemal K, Koffi A, Agnely F, Zhang M, Djabourov M, Ponchel G. Formulation of mucoadhesive vaginal hydrogels insensitive to dilution with vaginal fluids. Eur J Pharm Biopharm. 2010; 76: 296–303.
- Date AA, Shibata A, Goede M, Sanford B, La Bruzzo K, Belshan M, et al. Development and evaluation of a thermosensitive vaginal gel containing raltegravir + efavirenz loaded nanoparticles for HIV prophylaxis. Antiviral Res. 2012; 96: 430–436.
Cite this article: Karla PK (2013) Thermo Reversible Buffered Tenofovir Nano Gel for Vaginal Delivery. J Pharmacol Clin Toxicol 1(2): 1010.
Current Issue Vol.1.1
The Redox Brain and Nitric Oxide: Implications for Psychiatric Illness
Caroline E Wass1 and Ana Andreazza2,3*
Caroline E Wass1 and Ana Andreazza2,3*
Pharmacological Modulators of Endothelial Progenitor Cell Therapy: Implications for Treatment with Thiazolidinediones
Maria Korah1, Yagna PR Jarajapu2, Maria B. Grant3 and Ashay D. Bhatwadekar3*
Maria Korah1, Yagna PR Jarajapu2, Maria B. Grant3 and Ashay D. Bhatwadekar3*
Profile of Suspect Adverse Drug Reactions in a Teaching Tertiary Care Hospital
Shubhatara Swamy1, Bhanuprakash2, Pratibha Nadig1*, Muralimohan3 and Manjula Shetty1
Shubhatara Swamy1, Bhanuprakash2, Pratibha Nadig1*, Muralimohan3 and Manjula Shetty1
Enhancing the Potency of F508del Correction: A Multi-Layer Combinational Approach to Drug Discovery for Cystic Fibrosis
Emily F Kirby, Ashley S Heard and X Robert Wang*
Emily F Kirby, Ashley S Heard and X Robert Wang*
Leptin Up-Regulates HECTD1 to Promote Phosphoinositide Metabolism and Cell Migration and Invasion in Breast Cancer Cells
Qiaodan Zheng1,2#, Xiang Li2#, Manjula Sunkara3, Andrew J. Morris3, Wenyuan Wu1* and Cai Huang2,4*
Qiaodan Zheng1,2#, Xiang Li2#, Manjula Sunkara3, Andrew J. Morris3, Wenyuan Wu1* and Cai Huang2,4*
Copyright © 2013 JSciMed Central. All rights reserved.