|
J Pharmacol Clin Toxicol 1(2):1013.
Submitted: 14 November 2013; Accepted: 20 December 2013; Published: 30 December 2013
Research Article
Key Role of Carnosic Acid in the Anxiolytic-like Activity of Rosmarinus officinalis Linn. in Rodents
Ajay Kumar, Priyangi Agarwal, Anshul Shakya, Ajit Kumar Thakur, and Vikas Kumar*
Neuropharmacology Research Laboratory, Department of Pharmaceutics, Indian Institute of Technology (Banaras Hindu University), India
*Corresponding author: Vikas Kumar, Neuropharmacology Research Laboratory, Department of Pharmaceutics, Indian Institute of Technology (Banaras Hindu University), Varanasi-221 005, Uttar Pradesh, India. Tel: 91-542-6702742; Fax: 91-542-2368428; Email: [email protected]
Rosmarinus officinalis Linn. has several therapeutic applications in folk medicine and carnosic acid is one of the major chemical constituents in this plant. Standardized extract of Rosmarinus officinalis (ROE) and carnosic acid (CA) isolated from Rosmarinus officinalis were evaluated for anxiolytic-like activity. Methods used were elevated plus maze, open field and social interaction tests. Charles foster albino rats were treated with ROE at the doses of 30, 100 and 300 mg/kg/day and CA at the doses of 5, 15 and 45 mg/kg/day, p.o. for seven consecutive days respectively. In all tests, ROE and CA exhibited significant anxiolytic activity. ROE and CA significantly increased the level of 5-hydroxy tryptamine and dopamine concentration in rat brain while norepinephrine concentration was decreased. These results suggest that both ROE and CA has significant anxiolytic effect in dose dependent manner and qualitatively similar to standard anxiolytic agent lorazepam, which likely to regulated through monoaminergic system in the brain. These observations strongly suggest that carnosic acid seems to be major active constituent of RO for its observed anxiolytic activity in rodents.
Keywords: Rosmarinus officinalis; Carnosic acid; Anxiolytic; Brain monoamines
Anxiety is a universal human emotion. It alerts us to potential threats and motivates us to prepare for challenges. Anxiety is a disorder characterized by a broad range of symptoms including altered mood, emotions and feelings. Clinical anxiety is a chronic disease that can interfere significantly in the individual's life quality. Cognitive-behavioral therapy (CBT), behavior therapy (BT), and medication have been found to be efficacious for the treatment of anxiety however it often takes more than 5-8 treatment sessions to make the person panic free [1,2]. Moreover, conventional treatment modalities are hindered by adverse effects and produce a partial remission [3,4]. Plants have been used for some psychiatric disorders, like St. John's wort that is largely studied for the treatment of depression [5-7], and Kava-kava for anxiety [8]. Because of the limitations of the anxiolytic therapy, there has been renewed interest in other alternative therapies with medicinal plants, which may have comparable efficacy to prescription medications while lacking their severe side effects [9,10].
Rosmarinus officinalis Linn. (syn: Rosemary, family: Lamiaceae) has several therapeutic applications in folk medicine [11] in curing or managing a wide range of diseases. Rosmarinus officinalis L. is native to Europe, but has been cultivated in all Brazilian states. Some studies have reported that the extract of this plant exerts a number of pharmacological activities, such as hepatoprotective [12], antibacterial [13], antithrombotic [14], antiulcerogenic [15], diuretic [16], antidiabetic and antioxidant [17], antinociceptive [18] and anti-inflammatory [19]. An ethnopharmacological use of Rosmarinus officinalis in the treatment of depression, among other uses, was reported [20]. The most abundant phenolic diterpene compound in Rosemary leaves is carnosic acid [21,22], a labile abietane diterpene that undergoes an oxidative degradation and rearrangement cascade, producing a series of compounds, many with antioxidant activity [21,23], such as carnosol, rosmanol [24], rosmariquinone [25], and methyl carnosate [26]. Carnosic acid is a lipophilic antioxidant that scavenges singlet oxygen, hydroxyl radicals, and lipid peroxyl radicals, thus preventing lipid peroxidation and disruption of biological membranes [27]. Its radical scavenging activity is caused by the presence of two O-phenolic hydroxyl groups formed at C11 and C12 of the molecule [28].
Considering the therapeutic applications of Rosmarinus officinalis in folk medicine in the management of neurological and psychiatric disorders, the present study was planned to support scientifically for its traditional claim by using reverse pharmacology approach. In view of impaired monoamines level which is one of the major contributions to psychiatric disorders including anxiety [29-31], we conclude that the observed potential anxiolytic-like activity of Rosmarinus officinalis and carnosic acid likely to be due its effect monoaminergic system.
Animals
Adult Charles foster rats of either sex (3 males and 3 females in each group) weighing 180 ± 20 g were used for animal studies [32-35]. The animals were obtained from the Central Animal House, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India and were randomly distributed into different experimental groups. The rats were housed in polyacrylic cages with stainless steel wire lids and maintained under standard laboratory conditions (temperature 25 ± 1 °C) and relative humidity (50 ± 5%) with dark and light cycle (12:12 hr). The animals were fed with standard pellet diet and water ad libitum unless stated otherwise. Principles of laboratory animal care (NIH publication number # 85-23, revised in 1985) guidelines were followed. The experimental protocol was approved by the Central Animal Ethical Committee of the University (No. Dean/10-11/284 dated October 19, 2010).
Drugs and treatment groups
The extract was standardized to contain carnosic acid (10% w/w) and carnosol (1% w/w) by HPLC. Assay of the isolated carnosic acid was found to be 95% when compared with the standard by HPLC. The extract of Rosmarinus officinalis (ROE) and carnosic acid (CA) were suspended in 0.5% Tween 80 in distilled water [18]. These suspensions were administered orally at three different doses (30, 100 and 300 mg/kg/day) of ROE and (5, 15 and 45 mg/kg/day) of CA respectively, for 7 consecutive days (day 1 to day 7) based on our pilot study. Behavioral experiments were performed 1 hour after administration of last dose (7th day) as per methods described somewhere [32-35]. The suspension of extract was freshly prepared immediately before its administration. A control group received 0.5% Tween 80 in distilled water as vehicle. Lorazepam (1 mg/kg, p.o.) was used as standard anxiolytic agent.
Elevated plus maze
The elevated plus maze has been used as a model predictive of anxiolytic activity. The method of Pillow et al. was followed [36]. The plus-maze consists of two open arms, 50 × 10 × 40 cm, and two enclosed arms, 50 × 10 × 40 cm, with an open roof, arranged so that the two open arms are opposite to each other. The arms were connected to a central square, 10 × 10 cm, giving the apparatus shape of plus sign. The maze was kept in a dimly lit room and elevated 50 cm above the floor. Rats were placed individually in centre of the maze, facing an enclosed arm. Thereafter, number of entries and time spent on the open and enclosed arms were recorded during the next 5 min by a blinded observer.
Open field test
The method of Bronstein was followed [37]. The open field apparatus was made of plywood and consisted of squares of (61 × 61 cm). The entire apparatus was printed black except for 6m thick white lines, which divided the floor into 16 squares. Open field was lighted by a 16 w bulb focusing on the field from a height of about 100 cm. The entire room, except the open field, was kept dark during the experiment. Each animal was centrally placed in the test apparatus for 5 min and the following behavioral aspects were noted:
Ambulation: This was measured in terms of no. of squares crossed by the animal, Rearing: Number of times the animal stood on its hind limbs, Self grooming: Number of times the animal groomed facial region and licked/washed/scratched various parts of the body, Activity in centre: Number of central square crossed by the animal and Fecal dropping: Number of fecal droppings excreted during the period.
Social interaction test
The method of File was followed [38]. The rats were first housed individually for 5 days before testing. The apparatus used for the test was a wooden box (60 × 60 × 35 cm) with a solid floor and was placed in a dimly lit room. On day 6, the rats were placed individually in the box and given to 7.5 min familiarization sessions at 2 hr interval. On day 7 rats were paired on weight and sex basis and place in the box for 7.5 min. During this time total time spent by the rat pair in “social interaction”, included sniffing, grooming, kicking boxing, biting, and crawling under or over the partner, was recorded in real time scoring method by a neutral ‘blinded' observer.
Estimation of brain monoamines
Immediately after the last regimen, animals were sacrificed by decapitation. The brain was rapidly removed and left in the ice-cold isotonic saline for a few seconds to cool throughout, and then trimmed for consistent separation of the spinal cord. Simultaneous determination of dopamine (DA), 5-hydroxy tryptamine (5-HT) and norephinephrine (NE) in a single rat brain was done by the method described by Welch and Welch [39].
Statistical analysis
All values were expressed as mean ± standard error mean (SEM). Statistical significance between control and treatment groups was analyzed by one way analysis of variance (ANOVA) followed by Dunnett's multiple comparisons test. GraphPad Prism-6 software was used for all statistical analysis.
Elevated plus maze test
Elevated plus maze (EPM) test is based on the principle that exposure to an elevated and open arm leads to an approach conflict that is stronger than that evoked by exposure to an enclosed arms of the maze. These responses are increased by anxiogenic agent and reduced by anxiolytics [40]. Both Rosmarinus officinalis extract (ROE) and carnosic acid (CA) treated rats exhibited significant dose dependent decrease in time spent in enclosed arms [F (7, 40) = 8.96] and number of entries into enclosed arms [F (7, 40) = 16.91] compared to control group. Also there was significant dose dependent increase in time spent in open arms [F (7, 40) = 46.88] and numbers of entries into open arms [F (7, 40) = 15.73] compared to control group. The observed anxiolytics-like effect of Rosmarinus officinalis and carnosic acid was qualitatively analogous to standard drug lorazepam (Table 1).
 Table 1
Table 1 Effect of Rosmarinus officinalis extract and carnosic acid on elevated plus maze in rats. Values are mean ± SEM, n=6. ROE- Rosmarinus officinalis extract, CA- carnosic acid. *=p<0.05; **=p<0.01; ***=p<0.001 vs. vehicle control.
Table 1 Effect of Rosmarinus officinalis extract and carnosic acid on elevated plus maze in rats. Values are mean ± SEM, n=6. ROE- Rosmarinus officinalis extract, CA- carnosic acid. *=p<0.05; **=p<0.01; ***=p<0.001 vs. vehicle control.
×
Open field test
In open field test (OFT), when animals are taken from their home cage and placed in a novel environment, they express their anxiety and fear by decrease in ambulation and exploration freezing, rearing and grooming behaviors and increase in defecation due to heightened autonomic activity [41]. Likewise lorazepam treated animals; the ROE and CA treated rats were showed significant increase in open field ambulation [F (7, 40) = 86.19], activity in centre [F (7, 40) = 13.16], self grooming [F (7, 40) = 13.01] and rearing [F (7, 40) = 16.37] in comparison to vehicle treated rats indicating anxiolytic activity of Rosmarinus officinalis and carnosic acid. Additionally the fecal droppings were significantly decreased [F (7, 40) = 7.731] in dose dependent manner after treatment with both ROE and CA (Table 2).
 Table 2
Table 2 Effect of Rosmarinus officinalis extract and carnosic acid on elevated plus maze in rats.
Table 2 Effect of Rosmarinus officinalis extract and carnosic acid on elevated plus maze in rats.
×
Social interaction test
The SIT is based on the fact that, an increase in social interaction without a concomitant increase in motor activity is indicative of anxiolytic activity [42]. Rats treated with ROE and CA spent significantly more time in social interaction [F (7, 40) = 217.3] in comparison to control rats in a dose dependent manner. Likewise, lorazepam also caused significant increase in social interaction in rats and its effect were also found to be qualitatively comparable to that of Rosmarinus officinalis and carnosic acid (Figure 1).
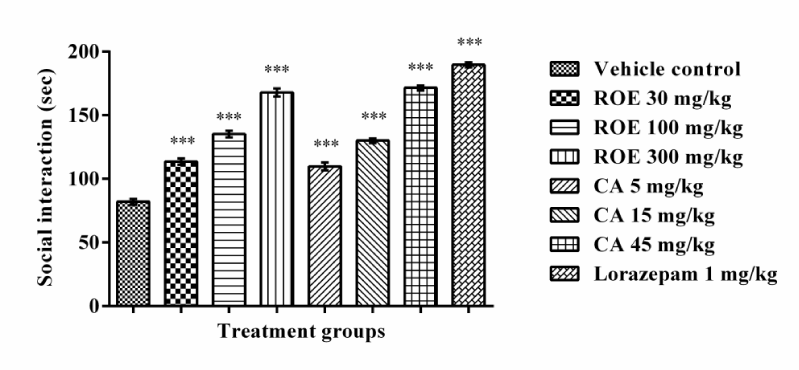 Figure 1
Figure 1 Effect of Rosmarinus officinalis extract and carnosic acid in social interaction test in rats. ROE- Rosmarinus officinalis extract, CA- carnosic acid. Values are mean ± SEM, n=6. ***=p<0.001 vs. vehicle control.
Figure 1 Effect of Rosmarinus officinalis extract and carnosic acid in social interaction test in rats. ROE- Rosmarinus officinalis extract, CA- carnosic acid. Values are mean ± SEM, n=6. ***=p<0.001 vs. vehicle control.
×
Level of brain monoamines
Impaired monoamines level is one of the major contributions to anxiety. Various classes of anxiolytics exert their effect by achieving any of the monoamines processing mechanisms like synthesis, release and synaptic availability. In anxiety majority of evidence supports the brain deficiency of serotonergic [29] and dopaminergic activity [30], while over activation of noradrenergic system [31]. In the present study ROE and CA produced significant increase in 5-HT [F (7, 40) = 4.507] and DA [F (7, 40) = 13.90] levels in brain while NE level [F (7, 40) = 39.75] was decreased in a dose dependent manner compared to control group and qualitatively similar to standard benzodiazepine drug (Figure 2).
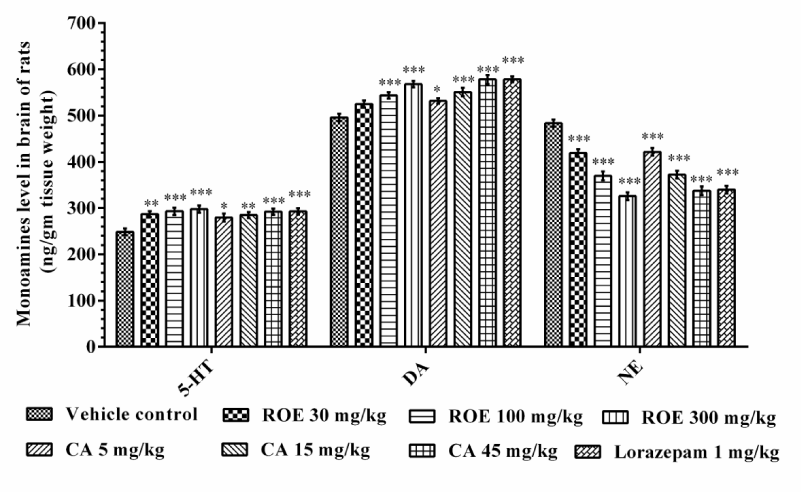 Figure 2
Figure 2 Effect of Rosmarinus officinalis extract and carnosic acid on the monoamines level in brain. ROE- Rosmarinus officinalis extract, CA- carnosic acid, 5-HT- 5-hydroxytreptamine, DA- dopamine, NE- norepinephrine, Values are mean ± SEM, n=6. *=p<0.05; **=p<0.01; ***=p<0.001 vs. vehicle control.
Figure 2 Effect of Rosmarinus officinalis extract and carnosic acid on the monoamines level in brain. ROE- Rosmarinus officinalis extract, CA- carnosic acid, 5-HT- 5-hydroxytreptamine, DA- dopamine, NE- norepinephrine, Values are mean ± SEM, n=6. *=p<0.05; **=p<0.01; ***=p<0.001 vs. vehicle control.
×
Many activities of the rosemary extracts were attributed to the carnosic acid content. So it is used for evaluation of its effect on the anxiety. Most of the animals models of anxiety now in use were developed for benzodiazepines (BDZ) and, since these compounds also exhibit significant muscle relaxant and anticonvulsant effects, evaluation of anxiolytic activity, even with non-BDZ compounds, invariably now includes test for these neuropharmacological actions [43]. The sedative, amnesic and ataxic effects of BDZ and non-BDZ anxiolytics are definite drawbacks when these drugs are used for the treatment of anxiety [36]. RO and its active chemical constituent CA have also reported to have antioxidant activity [17,27]. Oxidative stress involved in many acute and chronic diseases including cancer, cardiovascular disorders and neurodegenerative disease including anxiety. Bouayed et al. (2007) have studied the relationship between the level of intracellular reactive oxygen species in peripheral granulocytes and estimated anxiety level of rodents. The results of this study suggest a positive relationship between peripheral oxidative status and level of anxiety another study shows that a high anxiety level significantly increased the generation of reactive oxygen species in the rodents [31,44]. In view of this, the reported antioxidant activity of RO and CA is likely to play a significant role in our observed anxiolytic effect of ROE.
The findings of the present study indicate that ROE and CA treatment caused significant dose related anxiolytics-like activity in rats tested on all the behavior paradigms viz. EPM, OFT and SIT and the results were qualitatively similar to standard anxiolytic agent lorazepam. Further, observed anxiolytic effect of ROE and CA seems to be regulated through monoaminergic system in the brain.
Rosmarinus officinalis extract possesses promising anxiolytic activity regulated through monoaminergic system in the brain and carnosic acid is likely to be its active chemical constituent responsible for observed anxiolytic activity.
The financial assistance received from the University Grants Commission, New Delhi, India is thankfully acknowledged. Authors would like to thank Dr. Sushil Joshi (Ennature Biopharma-Indian Glycols Ltd., Dehradun, India) for the gift samples of standardized extract of Rosmarinus officinalis and isolated carnosic acid.
- Jesse W, Richardson J, Leonardo ED, Hen R, Ahmari S. Animal models of anxiety disorders: Behavioral and genetic approaches. In: Simpson HB, Neria Y, Fernandez RL, Schneier F, editors. Anxiety disorders, theory, research and clinical perspective. New York: Cambridge University Press. 2010; 156-67.
- Machado DG, Bettio LE, Cunha MP, Capra JC, Dalmarco JB, Pizzolatti MG, et al. Antidepressant-like effect of the extract of Rosmarinus officinalis in mice: involvement of the monoaminergic system. Prog Neuropsychopharmacol Biol Psychiatry. 2009; 33: 642-650.
- Páez-Pereda M. New drug targets in the signaling pathways activated by antidepressants. Prog Neuropsychopharmacol Biol Psychiatry. 2005; 29: 1010-1016.
- Taylor S, Stein MB. The future of selective serotonin reuptake inhibitors (SSRIs) in psychiatric treatment. Med Hypotheses. 2006; 66: 14-21.
- Nathan PJ. Hypericum perforatum (St John's Wort): a non-selective reuptake inhibitor? A review of the recent advances in its pharmacology. J Psychopharmacol. 2001; 15: 47-54.
- Rodriguez-Landa JF, Contreras CM. A review of clinical and experimental observations about antidepressant actions and side effects produced by Hypericum perforatum extracts. Phytomedicine. 2003; 10: 688-699.
- Sakakibara H, Ishida K, Grundmann O, Nakajima J, Seo S, Butterweck V, et al. Antidepressant effect of extracts from Ginkgo biloba leaves in behavioral models. Biol Pharm Bull. 2006; 29: 1767-1770.
- Bilia AR, Gallon S, Vincieri FF. Kava-kava and anxiety: growing knowledge about the efficacy and safety. Life Sci. 2002; 70: 2581-2597.
- Bilia AR, Gallori S, Vincieri FF. St. John's wort and depression: efficacy, safety and tolerability-an update. Life Sci. 2002; 70: 3077-3096.
- McGarry H, Pirotta M, Hegarty K, Gunn J. General practitioners and St. John's Wort: a question of regulation or knowledge? Complement Ther Med. 2007; 15: 142-148.
- Abu-Al-Basal MA. Healing potential of Rosmarinus officinalis L. on full-thickness excision cutaneous wounds in alloxan-induced-diabetic BALB/c mice. J Ethnopharmacol. 2010; 131: 443-450.
- Sotelo-Félix JI, Martinez-Fong D, Muriel P, Santillán RL, Castillo D, Yahuaca P. Evaluation of the effectiveness of Rosmarinus officinalis (Lamiaceae) in the alleviation of carbon tetrachloride-induced acute hepatotoxicity in the rat. J Ethnopharmacol. 2002; 81: 145-154.
- Del Campo J, Amiot MJ, Nguyen-The C. Antimicrobial effect of rosemary extracts. J Food Prot. 2000; 63: 1359-1368.
- Yamamoto J, Yamada K, Naemura A, Yamashita T, Arai R. Testing various herbs for antithrombotic effect. Nutrition. 2005; 21: 580-587.
- Dias PC, Foglio MA, Possenti A, de Carvalho JE. Antiulcerogenic activity of crude hydroalcoholic extract of Rosmarinus officinalis L. J Ethnopharmacol. 2000; 69: 57-62.
- Haloui M, Louedec L, Michel JB, Lyoussi B. Experimental diuretic effects of Rosmarinus officinalis and Centaurium erythraea. J Ethnopharmacol. 2000; 71: 465-472.
- Bakirel T, Bakirel U, KeleÅŸ OU, Ulgen SG, Yardibi H. In vivo assessment of antidiabetic and antioxidant activities of rosemary (Rosmarinus officinalis) in alloxan-diabetic rabbits. J Ethnopharmacol. 2008; 116: 64-73.
- Gonzalez-Trujano ME, Pena EI, Martinez AL, Moreno J, Guevara-Fefer P, Deciga-Campos M, et al. Evaluation of the antinociceptive effect of Rosmarinus officinalis L. using three different experimental models in rodents. J Ethnopharmacol. 2007; 111: 476-482.
- Altinier G, Sosa S, Aquino RP, Mencherini T, Della Loggia R, Tubaro A. Characterization of topical antiinflammatory compounds in Rosmarinus officinalis L. J Agric Food Chem. 2007; 55: 1718-1723.
- Heinrich M, Kufer J, Leonti M, Pardo-de-Santayana M. Ethnobotany and ethnopharmacology--interdisciplinary links with the historical sciences. J Ethnopharmacol. 2006; 107: 157-160.
- Richheimer SL, Bernart MW, King GA, Kent MC, Bailey DT. Antioxidant activity of lipid-soluble phenolic diterpenes from rosemary. J Am Oil Chem Soc. 1996; 73: 507-514.
- Aruoma OI, Halliwell B, Aeschbach R, Löligers J. Antioxidant and pro-oxidant properties of active rosemary constituents: carnosol and carnosic acid. Xenobiotica. 1992; 22: 257-268.
- Richheimer SL, Bailey DT, Bernart MW, Kent M, Vininski JV, Anderson LD. Antioxidant activity and oxidative degradation of phenolic compounds isolated from rosemary. Recent Res Dev Oil Chem. 1999; 3: 45-58.
- Schwarz K, Ternes W. Antioxidative constituents of Rosmarinus officinalis and Salvia officinalis. I. Determination of phenolic diterpenes with antioxidative activity amongst tocochromanols using HPLC. Z Lebensm Unters Forsch. 1992; 195: 95-98.
- Hall C, III, Cuppett S, Dussault P. Hydrogen-donating mechanism of rosmariquinone, an antioxidant found in rosemary. J Am Oil Chem Society. 1998; 75: 1147-1154.
- Cuvelier ME, Berset C, Richard H. Antioxidant constituents in sage (Salvia officinalis). J Agric Food Chem. 1994; 42: 665-669.
- Wenkert E, Fuchs A, McChesney JD. Chemical artifacts from the family Labiatae. J Org Chem. 1965; 30: 2931-2934.
- Munné-Bosch S, Alegre L. Subcellular compartmentation of the diterpene carnosic acid and its derivatives in the leaves of rosemary. Plant Physiol. 2001; 125: 1094-1102.
- Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety. 2000; 12 Suppl 1: 2-19.
- van der Wee NJ, van Veen JF, Stevens H, van Vliet IM, van Rijk PP, Westenberg HG. Increased serotonin and dopamine transporter binding in psychotropic medication-naive patients with generalized social anxiety disorder shown by 123I-beta-(4-iodophenyl)-tropane SPECT. J Nucl Med. 2008; 49: 757-763.
- Bouayed J, Rammal H, Younos C, Soulimani R. Positive correlation between peripheral blood granulocyte oxidative status and level of anxiety in mice. Eur J Pharmacol. 2007; 564: 146-149.
- Singh GK, Chauhan SK, Rai G, Chatterjee SS, Kumar V. Potential antianxiety activity of Fumaria indica: A preclinical study. Pharmacogn Mag. 2013; 9: 14-22.
- Kumar V. Characterization of anxiolytic and neuropharmacological activities of Silexan. Wien Med Wochenschr. 2013; 163: 89-94.
- Husain GM, Chatterjee SS, Singh PN, Kumar V. Beneficial effect of Hypericum perforatum on depression and anxiety in a type 2 diabetic rat model. Acta Pol Pharm. 2011; 68: 913-918.
- Thakur AK, Chatterjee SS, Kumar V. Anxiolytic-like activity of leaf extract of Traditionally used Indian-Mustard (Brassica juncea) in diabetic rats. TANG: Int J Genuine Tradit Med. 2013; 3: e7.1-e7.7.
- Pellow S, File SE. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacol Biochem Behav. 1986; 24: 525-529.
- Bronstein PM. Open-field behavior of the rat as a function of age: Cross-sectional and longitudinal investigations. J Comp Physiol Psychol. 1972; 80: 335-41.
- File SE. Animal models for predicting clinical efficacy of anxiolytic drugs: social behaviour. Neuropsychobiology. 1985; 13: 55-62.
- Welch AS, Welch BL. Solvent extraction method for simultaneous determination of norepinephrine, dopamine, serotonin, and 5-hydroxyindoleacetic acid in a single mouse brain. Anal Biochem. 1969; 30: 161-179.
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985; 14: 149-167.
- Bhattacharya SK, Satyan KS. Experimental methods for evaluation of psychotropic agents in rodents: I--Anti-anxiety agents. Indian J Exp Biol. 1997; 35: 565-575.
- File SE. The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. J Neurosci Methods. 1980; 2: 219-238.
- Wada T, Nakajima R, Kurihara E, Narumi S, Masuoka Y, Goto G, et al. Pharmacologic characterization of a novel non-benzodiazepine selective anxiolytic, DN-2327. Jpn J Pharmacol. 1989; 49: 337-349.
- Rammal H, Bouayed J, Younos C, Soulimani R. The impact of high anxiety level on the oxidative status of mouse peripheral blood lymphocytes, granulocytes and monocytes. Eur J Pharmacol. 2008; 589: 173-175.
Cite this article: Muhammad Amin OS (2014) Key Role of Carnosic Acid in the Anxiolytic-like Activity of Rosmarinus officinalis Linn. in Rodents. J Neurol Disord Stroke 2(2): 1013.
|